Laboratory high-purity water equipment
Reliable Solution for Analytical & Research Applications
Product Overview
Laboratory High-Purity Water Equipment is designed to produce consistently stable, high-quality pure and ultrapure water for laboratories, research centers, hospitals, and testing institutions.
The system combines reverse osmosis (RO), EDI, and polishing technologies to meet the strict water quality requirements of modern analytical and experimental applications.
It is suitable for supplying Type II and Type I laboratory water, ensuring accuracy, repeatability, and safety in laboratory operations.

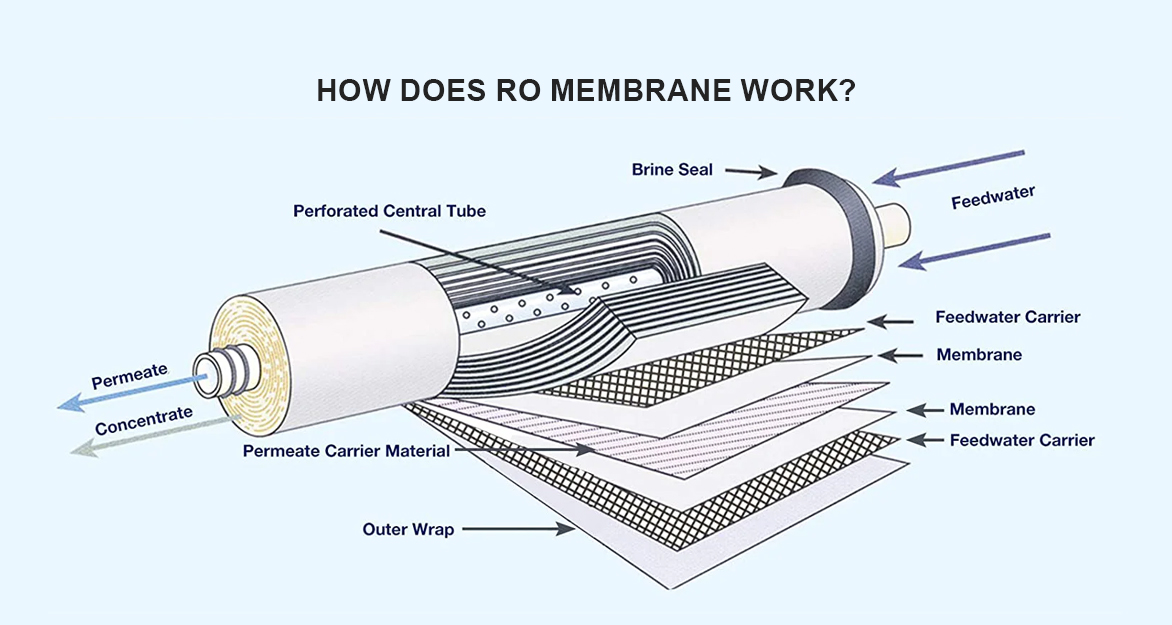
Working Principle
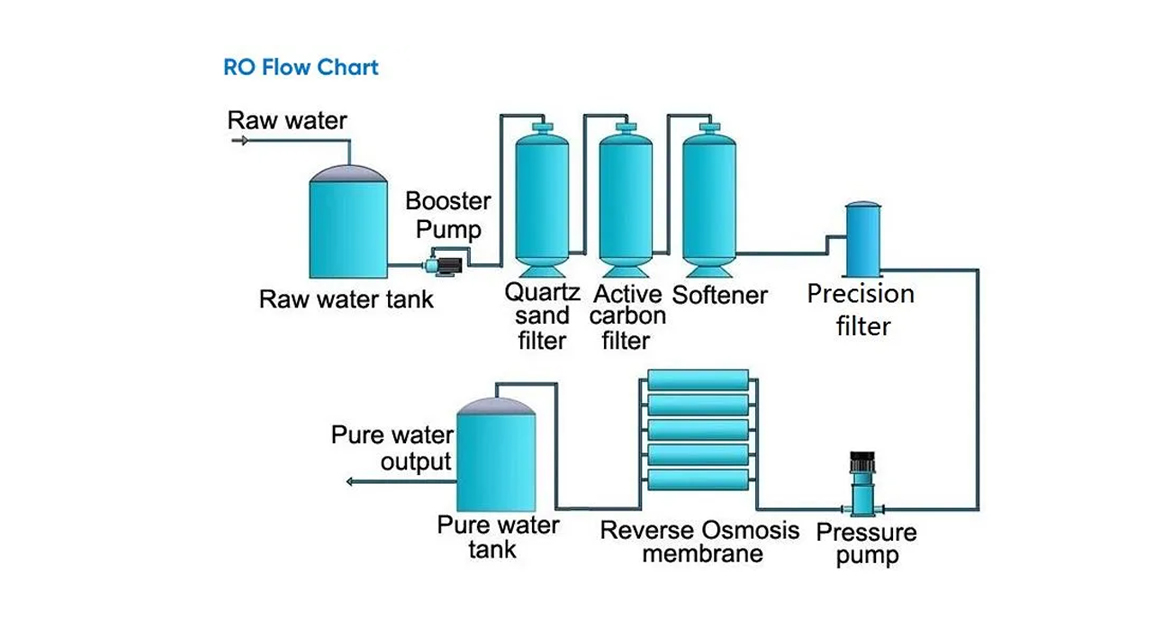
The system adopts a multi-stage purification process to remove impurities at different levels:
-
Pretreatment removes suspended solids, chlorine, and organic matter
-
Reverse Osmosis (RO) removes dissolved salts, heavy metals, bacteria, and endotoxins
-
EDI Module continuously removes residual ions without chemical regeneration
-
Polishing Unit (mixed bed resin, UV, ultrafiltration) ensures final ultrapure water quality
This design guarantees stable conductivity, low TOC, and low microbial content.
Key Features & Advantages
-
High purity and stable water quality
-
Chemical-free operation with EDI technology
-
Low TOC and endotoxin content
-
Compact design, ideal for laboratory installation
-
PLC automatic control & real-time monitoring
-
Low noise and energy-efficient operation
-
Modular expansion for multiple water points
System Configuration Options
-
Capacity: 10–1000 L/H
-
Water type: Type I / Type II / Type III
-
RO membranes: DuPont / Hydranautics / Vontron
-
EDI modules: Ionpure / Equivalent
-
Optional units: Online resistivity monitor, TOC analyzer, multiple water outlets

