Marine seawater desalination system
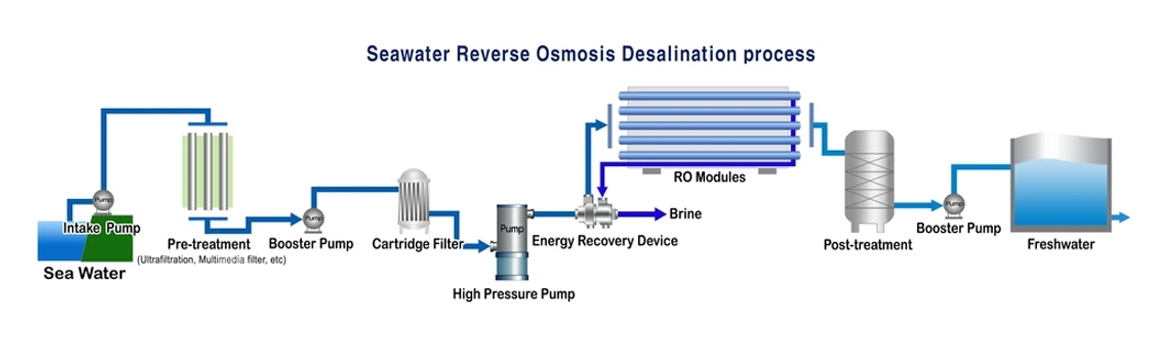
Membrane separation desalination, a method first introduced in 1953, separates seawater from freshwater using a semipermeable membrane that allows only the solvent to pass through, but not the solutes. Under normal circumstances, freshwater diffuses through the membrane into the seawater side, gradually raising the liquid level on the seawater side until it reaches a certain height. This process is called osmosis.
At this point, the static pressure of the water column on the seawater side is called osmotic pressure. If an external pressure greater than the seawater's osmotic pressure is applied to the seawater side, the pure water in the seawater will reverse osmosis into freshwater. The greatest advantage of reverse osmosis is energy efficiency. Its energy consumption is only half that of electrodialysis and 1/40 that of distillation. Therefore, since 1974, developed countries such as the United States and Japan have shifted their focus to reverse osmosis.
Equipment principle
A membrane that is selective in the substances it allows to pass through is called a semipermeable membrane. A membrane that is permeable only to solvents but not solutes is generally called an ideal semipermeable membrane. When equal volumes of a dilute solution (e.g., fresh water) and a concentrated solution (e.g., salt water) are placed on either side of a semipermeable membrane, the solvent in the dilute solution will naturally flow through the membrane toward the concentrated solution. This phenomenon is called osmosis. When osmotic equilibrium is reached, the liquid level on the concentrated solution side will be higher than that on the dilute solution side by a certain amount, creating a pressure differential known as the osmotic pressure.
The magnitude of osmotic pressure depends on the inherent properties of the solution, namely, the type, concentration, and temperature of the concentrated solution, and is independent of the properties of the semipermeable membrane. If a pressure greater than the osmotic pressure is applied to the concentrated solution, the solvent will flow in the opposite direction of the original osmotic flow, from the concentrated solution to the dilute solution. This process is called reverse osmosis.
Reverse osmosis is a reverse migration movement of osmosis. It is a separation method that separates the solute and solvent in the solution with the help of the selective interception effect of a semipermeable membrane under pressure. It has been widely used in the purification and concentration of various liquids. The most common application example is in the water treatment process, using reverse osmosis technology to remove impurities such as inorganic ions, bacteria, viruses, organic matter and colloids in raw water to obtain high-quality pure water.
Equipment features
Reverse osmosis desalination technology is developing rapidly, and project costs and operating costs continue to decrease. The main development trends are to reduce the operating pressure of reverse osmosis membranes, improve the recovery rate of reverse osmosis systems, use cheap and efficient pretreatment technologies, and enhance the system's anti-pollution capabilities.
Covered industries
Membrane filtration is widely used in the fields of electricity, petrochemicals, steel, electronics, medicine, food and beverages, municipal administration and environmental protection. It plays an important role in the desalination of seawater and brackish water, the preparation of boiler feed water, industrial pure water and electronic grade ultrapure water, the production of drinking pure water, wastewater treatment and special separation processes.