Chlorine dioxide generator
High-Efficiency & Safe Disinfection System
Product Overview
Chlorine Dioxide Generator is an on-site disinfection system designed to generate chlorine dioxide (ClO₂) for water treatment applications.
Chlorine dioxide is a highly effective broad-spectrum disinfectant, capable of rapidly killing bacteria, viruses, spores, and algae without producing harmful chlorinated by-products.
It is widely used in drinking water treatment, wastewater treatment, industrial process water, medical facilities, food processing, and municipal disinfection systems.
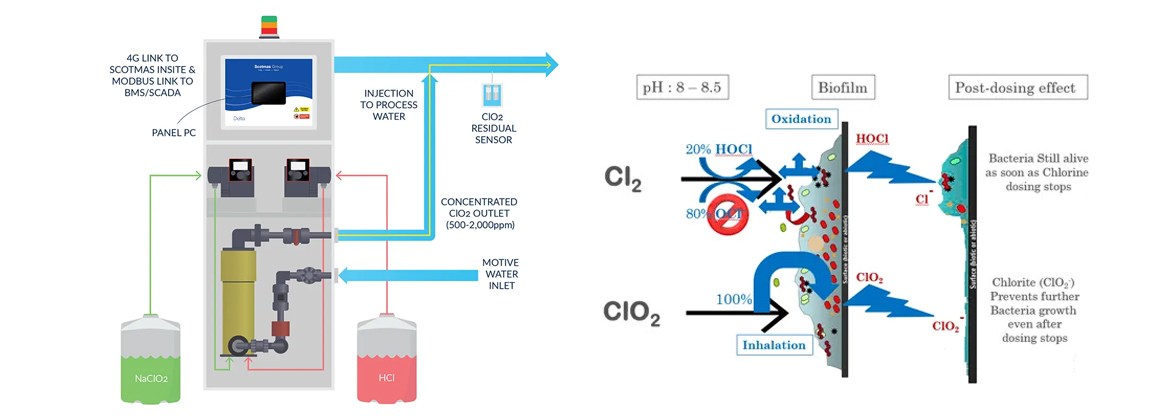
Working Principle
-
Raw materials (sodium chlorite and activator) are precisely dosed
-
Chemical reaction generates chlorine dioxide gas
-
ClO₂ is dissolved into water to form a disinfectant solution
-
The solution is injected into the water system for sterilization
The system operates automatically, safely, and continuously.

Key Features & Advantages
-
Strong sterilization ability
-
Broad-spectrum disinfection
-
Effective at low dosage
-
No secondary pollution
-
No trihalomethane formation
-
On-site generation for high safety
-
Automatic control & stable output

Application Fields
-
Drinking water disinfection
-
Municipal wastewater treatment
-
Industrial circulating water
-
Hospitals & medical water systems
-
Food & beverage processing
-
Cooling water & pipeline sterilization
Optional Configurations
-
PLC control cabinet
-
Online residual chlorine monitoring
-
Automatic dosing system
-
Skid-mounted integrated design
Reliable Disinfection Equipment
Chlorine dioxide generators provide efficient, stable, and environmentally friendly disinfection, making them an ideal solution for modern water treatment systems requiring high safety and performance.

